This is the first part in a series I introduced earlier where I will go into detail about my specific experiences with specific examples.
With the Advanced Technologies & Treatments for Diabetes 2025 conference in Amsterdam taking part this week, it seemed fitting to start off the series with Technology.
Side note – while I am not in attendance at the ATTD conference, I am hoping to have highlights from the conference as representatives from my employer presented and I’m hoping there will be some debriefings and summaries that I may be able to share with you.
Today, however, I’m specifically going to cover continuous glucose monitors, or CGMs.
A CGM simply, is a wearable sensor that inserts a fine gage needle into the interstitial fluid just below the skin. CGMs do not measure blood glucose in the same way as a traditional BG meter. The blood glucose meter uses a finger stick to expose blood directly to a sensing strip that gives a readout of blood glucose. A CGM uses the interstitial fluid that exists in the spaces between cells, a substance that leaks from blood capillaries. It’s chock full of biomarkers making it capable of providing a glucose readout similar to a BG meter.
However, because it is not collecting data directly from blood, CGMs are at a slight disadvantage and can have accuracy issues. That’s going to be my primary focus today.
The pros of CGMs are significant. The value of seeing real time changes in glucose levels is immeasurable. Clinicians are able to make more informed diagnoses and provide more finely tuned therapies using the real time data and a short term mean glucose concentration metric known as the Glucose Management Indicator, or GMI. This gives it an advantage over the gold standard for assessing glycemic control, the hbA1C, but is not a replacement as there can be clinically significant differences in the two values. If you’re really interested in getting into the weeds about GMI and A1C, I recommend this article from the journal Diabetes Care.
Patients benefit from continuous glucose monitoring as well. The ability to observe glycemic fluctuation can inform decisions regarding diet and exercise, which have real implications for treatment strategies.
As an ultra endurance athlete, this data provides important insights into many of the decisions I have to make in-race which can determine whether or not I finish. Making sure to take in calories during an endurance effort is paramount and obvious but with most products designed for people without metabolic disorders choosing safe and effective sources of energy is a challenge. The live readout of glycemic fluctuations during a race combined with the anecdotal experience of consuming fluid, goops, or other foods can shape future strategies.
There are a number of options to choose from though there are only 4 major manufacturers that are FDA approved. The two largest players are Dexcom and Medtronics. The next two companies are Abbott and Senseonics. We’re going to compare the flagships of Dexcom and Abbott later in this post as those are the devices that I have personal experience with, but I want to highlight an interesting thing about the Senseonics device, Eversense, which is the only implantable CGM on the US market and is advertised to last 90 days. This makes it a very intriguing entry into the space as the Abbott and Dexcom devices only last 15 and 10 days respectively. A company called GlucoTrack is currently in human clinical trials with their own implantable, continuous blood glucose meter. That will be interesting to keep tabs on.
For the purposes of this post, we will be ignoring Medtronics as their commercial CGM is integrated into an insulin pump and only available to individuals living with Type-1 Diabetes.
But let’s get back to that accuracy point I brought up. Much like the reliability gap between an optical heart rate monitor and one that measures electrical signals, interstitial fluid measurements are likely not going to be as reliable as measurements taken from blood samples.
This isn’t a secret, manufacturers of CGMs advertise their accuracy with a percentage score called MARD – median absolute relative difference. In short, MARD compares values collected from a CGM and a reference BG meter and outputs a difference over a large sample size. The industry and medical experts widely accept that any MARD below 10% is considered to be quite accurate.
Abbott advertises their flagship CGM, the Freestyle Libre 3 as the industry leader with a MARD of 7.9%
Dexcom, on the other hand, advertises the G7 has a MARD of 8.2%
I recently performed a comparative study that found interesting results that differed than advertised. I will admit, my sample size is probably inadequate as I was only able to collect data for the lifespan of a single Dexcom G7 (10 days). I do plan on expanding this data at some point and revisiting this comparison.
I was able to take 17 readings from a Libre 3 worn on my left arm, a G7 worn on my right arm, and a Abbott Freestyle Precision Neo BG meter used as the reference standard. I took both fasting and post prandial measurements as well is before and after extensive activity.
Here is what I found:
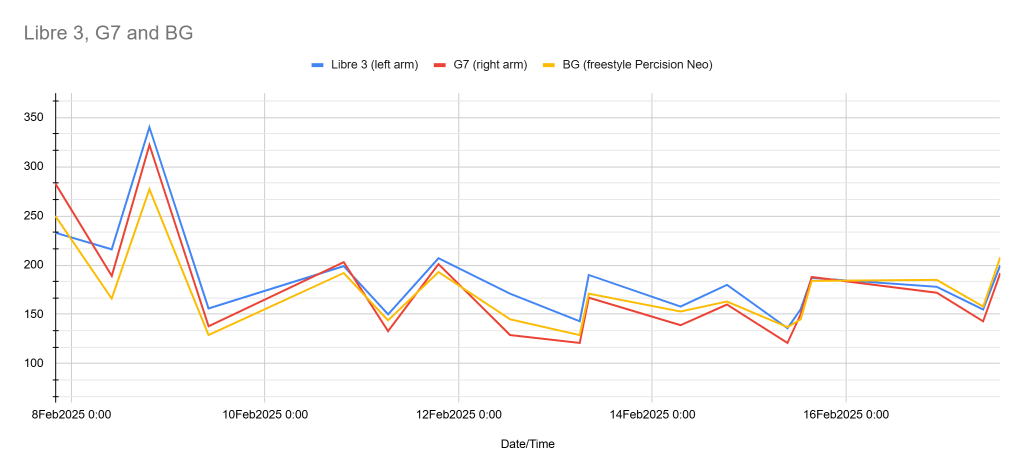
The Dexcom G7 was frequently lower than BG and the Freestyle Libre 3 was typically higher than BG.
The overall observed MARD calculations actually flipped the script. The G7 was more accurate than the Libre 3 and both were significantly off the mark of what they advertise
| Libre 3 (Target 7.9%) | G7 (Target 8.2%) | |
| Observed Overall MARD | 9.66% | 7.78% |
Intrigued by these results I actually broke it down further.
Surprisingly to me, the accuracy of both sensors improved after eating and worsened significantly when fasting. The Libre 3 even fell out of 10%. I would have thought it might be the other way around, that higher glucose levels would lead to further inaccuracies. This was the pattern I had observed anecdotally when using earlier models from both companies. The Libre 2 and the G6 seems to get less accurate the higher the BG reference was.
| Libre 3 (Target 7.9%) | G7 (Target 8.2%) | |
| Observed Overall MARD | 9.66% | 7.78% |
| MARD after Fasting (7 Samples) | 10.28% | 9.29% |
| Postprandial MARD (10 Samples) | 9.22% | 6.72% |
This made me curious about the performance of the sensor when the blood glucose reference was in range versus hyperglycemic, so I isolated the sample readings into the following categories.
| Libre 3 | G7 | |
| Observed MARD Over 180mg/dL | 2.70% | 2.85% |
This data is a little bit more suspect just due to sample size. Of the 17 total readings only 7 measurements with the Precision Neo were above 180 mg/dL however, the CGMs read above 180 mg/dL in just 6 of these instances. Similarly, of the in-range measurements (70-180 mg/dL) there were only 9 of 11 instances where both CGMs read in range at the same time as the reference BG meter.
| Libre 3 | G7 | |
| Observed In Range MARD | 6.96% | 4.92% |
To me this throws considerable doubt on the validity of the results as I have calculated them and I’d likely need to revisit my methodology. But even so, the trends that can be extrapolated may still be valid. When blood glucose is higher, both sensors appear to perform the same and when BG is in-range the G7 is substantially more accurate than the Libre 3.
There is likely going to be additional comparative studies done. I do have some G7s still and currently use the Libre 3. One of the additional things I want to explore is if activity influences accuracy. I have only collected one pre/post reading:
| Libre 3 | G7 | BG | |
| 15Feb 2025 12:45 Prior to Run | 155 | 150 | 145 |
| 15Feb2025 15:30 After 90 min aerobic threshold, a 19g UCAN gel, 30 min aerobic cooldown | 187 | 188 | 184 |
| MARD | 4.26% | 2.81% |
It’s clearly not enough to draw any conclusions but I’m eager to gather more pre/post data because I have often found that post exercise BG is higher due to release of glycogen. I think my next experiment will be similar to this comparative MARD study but will have a secondary goal of tracking immediately pre/post exercise and then again 2 hours after exercise both with and without eating.
It’s a more ambitious long term project so stay tuned for that much later in the year! I think I said this was just going to be a 3 part series but I suspect it will be more than that. We didn’t even tough on GPS/smart watches, smart scales, or sleep trackers!




Leave a reply to DT&G Part 1 (actually Part 2): Technology Continued – Type 4 Fun! Cancel reply